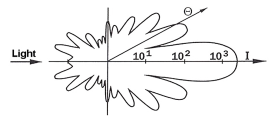
Mie Scattering
Mie scattering is elastic scattered light of particles that have a diameter similar to or larger than the wavelength of the incident light. The Mie signal is proportional to the square of the particle diameter. Mie scattering is much stronger than Rayleigh scattering and, therefore, a potential source of interference for this weaker light scattering process. There is a strong angular dependency of the scattered intensity especially for smaller particles which has to be considered for successful Mie imaging experiments. Mie scattering is often used to measure flow velocities applying Particle Image Velocimetry (PIV).
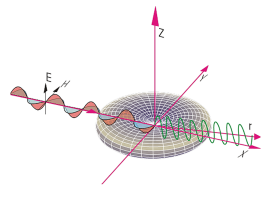
Rayleigh Scattering
Rayleigh scattering is the elastic scattering of light by particles much smaller than the wavelength of the light. That is the case for gas phase molecules and, therefore, this method is suited for laser imaging in gases. Rayleigh scattering of sunlight by atmospheric molecules is the reason for the observed blue color of the sky, because the scattering efficiency varies inversely with the fourth power of the wavelength. For a single component gas with known scattering cross section the Rayleigh signal is directly proportional to the gas density. The scattered light is almost at the same wavelength as the incident light, i. e. Rayleigh scattering is not species selective. Rayleigh scattering requires either constant gas composition or known mole fractions of all major species for the density measurement of a gas mixture. In some cases Rayleigh scattering is stronger for one species than another, and it can be used to image mixing processes such as fuel – air mixing.
When gas composition and pressure are known Rayleigh imaging allows to measure planar temperature fields (Rayleigh Thermometry). Rayleigh scattering is much weaker than Mie scattering but more than two orders of magnitude stronger than Spontaneous Raman Scattering. Incandescence from soot and Mie scattering are processes that can totally obscure the Rayleigh signal.
Raman Scattering
Spontaneous Raman Scattering is the inelastic counterpart to Rayleigh scattering. Raman scattering shows a spectral response that is shifted from the laser line. This shift is characteristic for the Raman active molecules and allows to measure all major species concentrations at the same time. Major gas phase constituents like O2, N2, hydrocarbon fuels, CO2, H2 and H2O can be measured simultaneously together with the gas temperature. The drawback of Raman scattering in the gas phase is the weakness of the signal, roughly six orders of magnitude less than Mie scattering. For 1D Raman imaging along a line focus multiple laser shots are averaged and high power laser are used. Raman like Rayleigh signals scale linearly with the molecular species' density and do not suffer from collisional quenching effects involved in LIF processes.

